
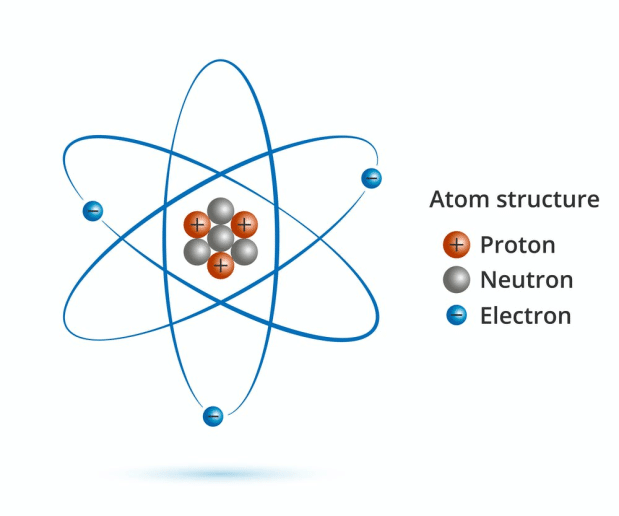
If the positive alpha particles mostly passed through the foil, but some bounced back. How could that be if the plumb pudding model was correct? Rutherford's experiment prompted a change in the atomic model. In 1922, the American physicist Arthur H. Rutherford found that most of them went right through the foil. The Modern Atomic Model The development of quantum mechanics served as the foundation of the modern atomic theory. Each of these models had advantages and disadvantages of its own and played a significant role in the development of the modern atomic model. The model proposed by James Chadwick focuses on the modeling of the atomic nucleus constituted not only by protons (positive charges), but also by neutrons. I am going to include a diagram for each of these models. I am going to give a brief history of the atomic model including Thomsons atom, Rutherfords atom, Bohrs atom, and Schrödingers atom. If you shoot these positive alpha particles at this positive pudding atom, they should mostly bounce off, right? Well, that is not what happened. Many scientists used atomic models to understand the structure of the atom in the early centuries. I am going to discuss the modern concept of the atom. He shot some alpha particles (which are really just the nucleus of a helium atom) at some really thin gold foil. Ernest Rutherford said one day "hey, I think I will shoot some stuff at atoms." I am sure his wife said "oh, Ernie" (she probably called him Ernie) "if it makes you happy to play with your little physics stuff, go ahead. 5 Modern Atomic Theory The Atom The Atom Video Transcript The Atom A neutral atom has the same number of protons as electrons.


 0 kommentar(er)
0 kommentar(er)
